chemical test to distinguish between aldehydes and ketones Difference between aldehyde and ketone
Today, we will dive into the fascinating world of aldehydes and ketones. Although these compounds may sound complex, they play a crucial role in various chemical reactions and are fundamental in organic chemistry.
Oxidation of Aldehydes and Ketones
 One remarkable characteristic of aldehydes and ketones is their ease of oxidation. The oxidation process involves the loss of electrons, resulting in the formation of new chemical bonds. This transformation can have significant implications in various chemical reactions.
One remarkable characteristic of aldehydes and ketones is their ease of oxidation. The oxidation process involves the loss of electrons, resulting in the formation of new chemical bonds. This transformation can have significant implications in various chemical reactions.
During oxidation, aldehydes are transformed into carboxylic acids, while ketones are converted into tertiary alcohols. This difference in behavior is primarily due to the presence of a hydrogen atom attached directly to the carbonyl carbon in aldehydes. This hydrogen atom is readily available to be transferred during the oxidation process, resulting in the formation of a carboxylic acid group.
The oxidation of aldehydes can be achieved through different methods, including the use of oxidizing agents such as potassium permanganate or chromic acid. These agents facilitate the transfer of oxygen atoms to the aldehyde, leading to the formation of a carboxylic acid. This transformation is particularly useful in the synthesis of various organic compounds, including pharmaceuticals.
On the other hand, the oxidation of ketones is more challenging due to the absence of a hydrogen atom on the carbonyl carbon. However, it is still possible to achieve oxidation by using more powerful oxidizing agents like nitric acid or acidic dichromate solutions. These agents allow for the breaking of carbon-carbon bonds adjacent to the carbonyl group, leading to the formation of tertiary alcohols.
Difference Between Aldehyde and Ketone
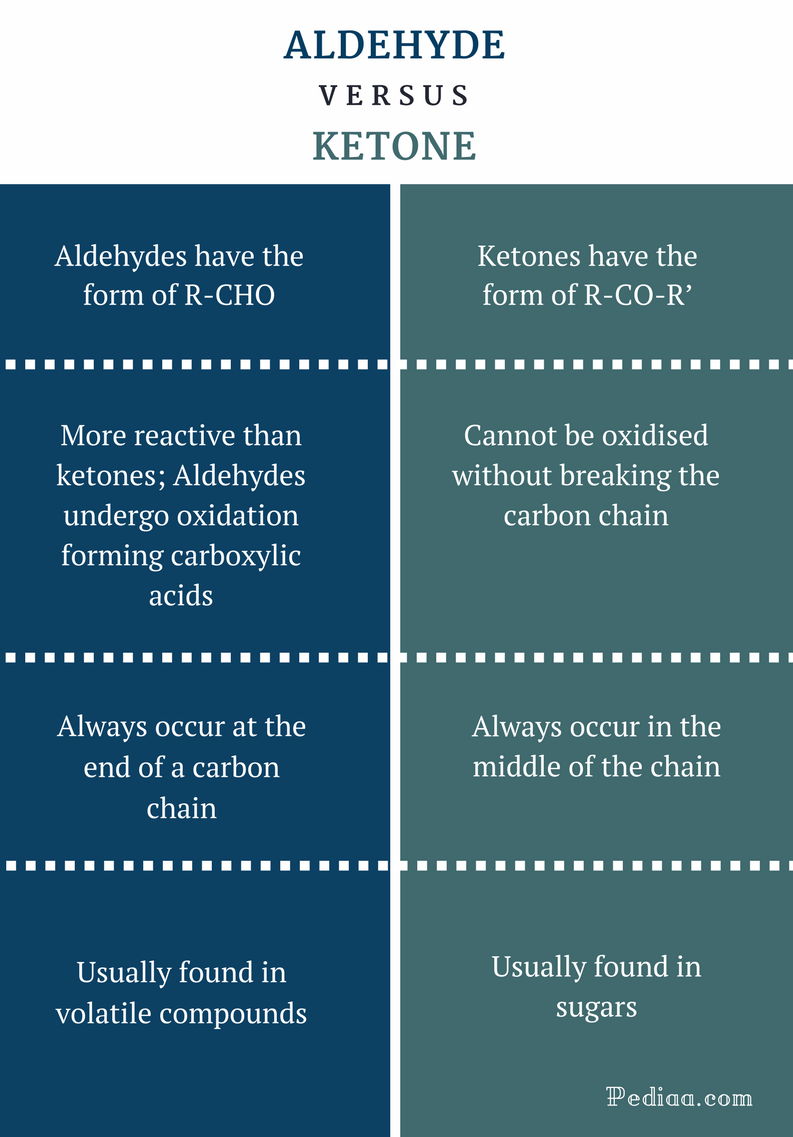 While aldehydes and ketones both contain carbonyl groups, they differ in their structural and chemical properties. Understanding these differences is essential for the accurate identification and classification of organic compounds.
While aldehydes and ketones both contain carbonyl groups, they differ in their structural and chemical properties. Understanding these differences is essential for the accurate identification and classification of organic compounds.
The main distinction between aldehydes and ketones lies in the location of the carbonyl group within the molecular structure. Aldehydes have the carbonyl group attached to a terminal carbon atom, whereas ketones have the carbonyl group situated on an internal carbon atom.
Another distinguishing feature is their naming conventions. Aldehydes are named by replacing the “-e” ending of the corresponding parent alkane with the suffix “-al.” In contrast, ketones are named by replacing the “-e” ending of the corresponding parent alkane with the suffix “-one.”
Furthermore, the reactivity of aldehydes and ketones varies due to the presence or absence of the hydrogen atom attached to the carbonyl carbon. This difference in reactivity also leads to variations in their physical properties, such as boiling points and solubility.
In conclusion, aldehydes and ketones are essential compounds in organic chemistry. Their distinct characteristics, such as ease of oxidation and differences in structure and properties, make them valuable building blocks for the synthesis of numerous organic compounds. Understanding these compounds enables chemists to develop new drugs, materials, and technologies that benefit various industries.
If you are looking for Give a Simple Chemical Test to Distinguish Between - Chemistry you’ve visit to the right place. We have 5 Pictures about Give a Simple Chemical Test to Distinguish Between - Chemistry like oxidation of aldehydes and ketones, Difference between Aldehyde and Ketone | Ketones, Chemistry notes and also Difference Between Aldehyde and Ketone | Structure, Properties, Naming. Here it is:
Give A Simple Chemical Test To Distinguish Between - Chemistry
 www.shaalaa.comchemical distinguish shaalaa ketones
www.shaalaa.comchemical distinguish shaalaa ketones
Ketones & Aldehydes Test

Oxidation Of Aldehydes And Ketones
 chemguide.ukaldehydes ketones oxidation aldehyde hydrogen bond reactions ketone difference between why reducing oxidise presence makes oxygen carbonyls double atom reduction
chemguide.ukaldehydes ketones oxidation aldehyde hydrogen bond reactions ketone difference between why reducing oxidise presence makes oxygen carbonyls double atom reduction
Difference Between Aldehyde And Ketone | Ketones, Chemistry Notes
 www.pinterest.comaldehyde ketone between ketones aldehydes
www.pinterest.comaldehyde ketone between ketones aldehydes
Difference Between Aldehyde And Ketone | Structure, Properties, Naming
 pediaa.comaldehyde ketone ketones pediaa aldehydes aldehyd keton carboxylic zwischen unterschied
pediaa.comaldehyde ketone ketones pediaa aldehydes aldehyd keton carboxylic zwischen unterschied
Aldehyde ketone ketones pediaa aldehydes aldehyd keton carboxylic zwischen unterschied. Difference between aldehyde and ketone. Ketones & aldehydes test